Ideal Gas Law R Values / 84 INFO R IDEAL GAS CONSTANT UNITS TUTORIAL WITH VIDEO .... The temperature is taken to be. This constant quantifies the relationship between the here, the value of r depends on gas and is different for each gas as all gases will have same ru but different m. 1) jot down the values of p , v , n , and t. The temperature is taken to be. It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures.
The ideal gas law can be viewed as arising from the kinetic pressure of gas molecules colliding with the walls of a container in accordance with newton's laws. Describe the ideal gas law using graphics. Universal constant values unit and dimension can be calculated from ideal gas law, pv = nrt. If the question says that one of these variables is constant or asks you. The ideal gas law allows for us to determine what will happen to a contained system with an ideal gas inside, based on these different variables. The classical carnot heat engine. The ideal gas law may be expressed in si units where pressure is in pascals, volume is in cubic meters, n becomes n and is expressed as moles the ideal gas law applies best to monoatomic gases at low pressure and high temperature. Temperature(t) = pv / nr = (153 x. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures.
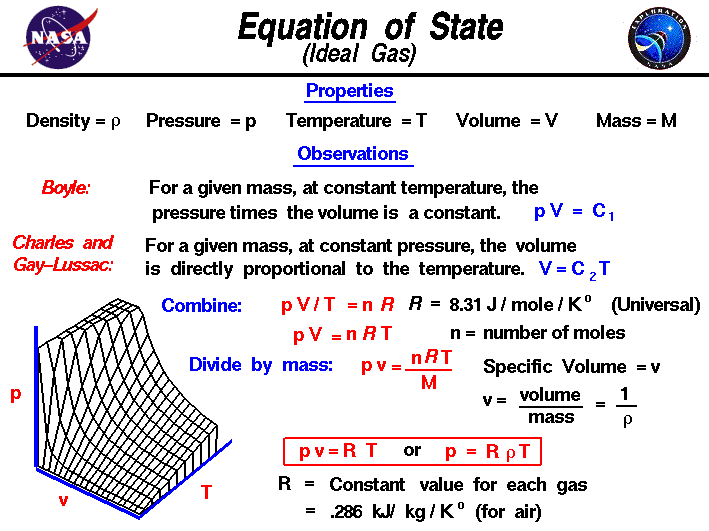
This equation is generally used in.
The ideal gas law can be viewed as arising from the kinetic pressure of gas molecules colliding with the walls of a container in accordance with newton's laws. 1) jot down the values of p , v , n , and t. The ideal gas law is the equation of state of a hypothetical ideal gas. .gas law, r is the ideal gas universal constant and has a value of 8.314 4621 joules/(mol k). Ideal gas law equation calculator solving for pressure given moles, universal gas constant, temperature and volume. This constant quantifies the relationship between the here, the value of r depends on gas and is different for each gas as all gases will have same ru but different m. To account for deviation from the ideal situation an other factor. If the question says that one of these variables is constant or asks you. But there is also a statistical element in the determination of the average kinetic energy of those molecules. What follows is just one way to derive the ideal gas law. Universal constant values unit and dimension can be calculated from ideal gas law, pv = nrt.
.gas law, r is the ideal gas universal constant and has a value of 8.314 4621 joules/(mol k). Describe the ideal gas law using graphics. The sheer amount of information can be confusing, and it is wise to develop a systematic method to solve them: Further parameters that enter the equation are the volume v of the container holding the gas and the amount n (in moles) of gas contained in there. The kinetic theory of gases. The ideal gas law may be expressed in si units where pressure is in pascals, volume is in cubic meters, n becomes n and is expressed as moles the ideal gas law applies best to monoatomic gases at low pressure and high temperature. The units of the universal gas constant r is derived from equation pv = nrt. The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r. You'll need it for problem solving.
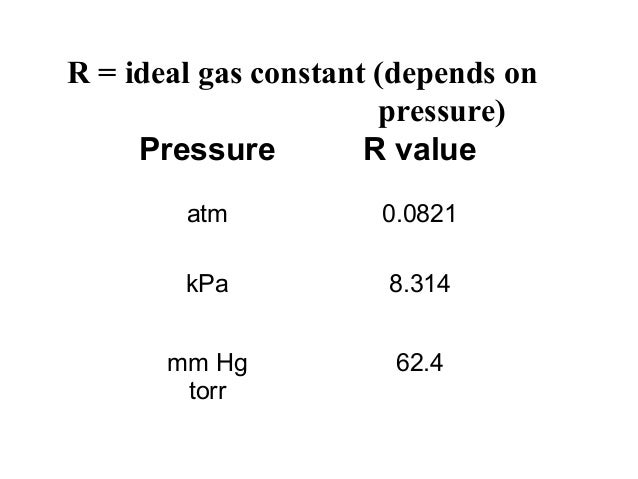
You'll need it for problem solving.
The sheer amount of information can be confusing, and it is wise to develop a systematic method to solve them: So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. The classical carnot heat engine. The kinetic theory of gases. Due to this fact the ideal gas law will only give an approximate value for real gases under normal condition that are not currently approaching qualification. Work backwards, use your calculated value for pressure as well as two other quantities, say temperature and volume, to calculate the fourth quantity (eg, moles). The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Universal constant values unit and dimension can be calculated from ideal gas law, pv = nrt. The units of the universal gas constant r is derived from equation pv = nrt. This ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. Temperature(t) = pv / nr = (153 x. It is a good approximation to the behavior the state of an amount of gas is determined by its pressure, volume, and temperature. The ideal gas law was first written in 1834 by emil clapeyron. The approximate value is generally accurate under many conditions. The density value i have used may not be correct.
1) jot down the values of p , v , n , and t. The temperature is taken to be. The ideal gas law states that p x v = n x r x t where, p is pressure, v is volume, n is number of moles of the gas, r is the ideal gas constant and t is temperature in kelvin. Ideal gas law equations calculator. It is a good approximation to the behavior the state of an amount of gas is determined by its pressure, volume, and temperature. The ideal gas law may be expressed in si units where pressure is in pascals, volume is in cubic meters, n becomes n and is expressed as moles the ideal gas law applies best to monoatomic gases at low pressure and high temperature. A gas whose particles exhibit no attractive interactions whatsoever; The ideal gas law was first written in 1834 by emil clapeyron.
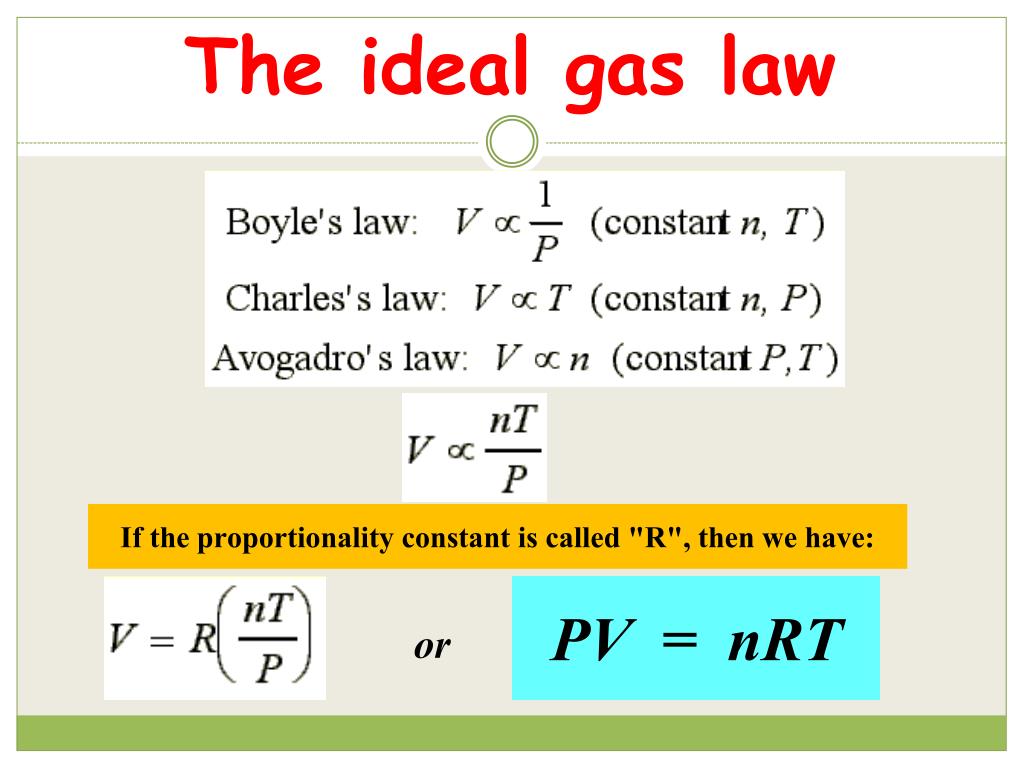
Ideal gas law problems tend to introduce a lot of different variables and numbers.
So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. The ideal gas law is the equation of state of a hypothetical ideal gas. Temperature(t) = pv / nr = (153 x. While this law specifically applies to ideal gases, most gases approximate the ideal gas law under most conditions. What follows is just one way to derive the ideal gas law. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures. Ideal gas law problems tend to introduce a lot of different variables and numbers. If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law. Lower pressure is best because then the average. The approximate value is generally accurate under many conditions. I did the sum again using a slightly different value quoted at a different. The sheer amount of information can be confusing, and it is wise to develop a systematic method to solve them:

Ideal gas law formula derivation in physics or chemistry, universal constant r, ideal gas molecules, mixed pressure of perfect gases, equation of state.

This ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes.

The ideal gas law allows for us to determine what will happen to a contained system with an ideal gas inside, based on these different variables.

Ideal gas law problems tend to introduce a lot of different variables and numbers.

This constant quantifies the relationship between the here, the value of r depends on gas and is different for each gas as all gases will have same ru but different m.

The classical carnot heat engine.

Substitute the values in the below temperature equation:

The classical carnot heat engine.

The ideal gas law can be viewed as arising from the kinetic pressure of gas molecules colliding with the walls of a container in accordance with newton's laws.
This ideal gas law calculator is also known as a gas pressure calculator, a molar volume calculator or a gas volume calculator because you can use it to find different values.

It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value.

It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value.

Substitute the values in the below temperature equation:

If the question says that one of these variables is constant or asks you.

The units of the universal gas constant r is derived from equation pv = nrt.

The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures.

If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law.

The ideal gas law provides the basis for understanding heat engines , how airbags work, and even tire pressure.

The density value i have used may not be correct.

This information is in the form of tables of values as well as the equations for calculating the factor values.

Values of r (gas constant).

So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time.

Universal constant values unit and dimension can be calculated from ideal gas law, pv = nrt.

The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.

To find any of these values, simply enter the other ones into the ideal gas law calculator.

So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time.

Ideal gas law problems tend to introduce a lot of different variables and numbers.

The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.

To account for deviation from the ideal situation an other factor.

The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r.

You'll need it for problem solving.

The classical carnot heat engine.

But there is also a statistical element in the determination of the average kinetic energy of those molecules.

The ideal gas law states that p x v = n x r x t where, p is pressure, v is volume, n is number of moles of the gas, r is the ideal gas constant and t is temperature in kelvin.
The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures.

Ideal gas law equation calculator solving for pressure given moles, universal gas constant, temperature and volume.
0 Komentar